Delivering What Mattersto Biopharma
Comprehensive Cell & Gene Therapy (CGT) Clinical Development Services

CGT Projects
CGT Professionals
CGT Leaders Experience
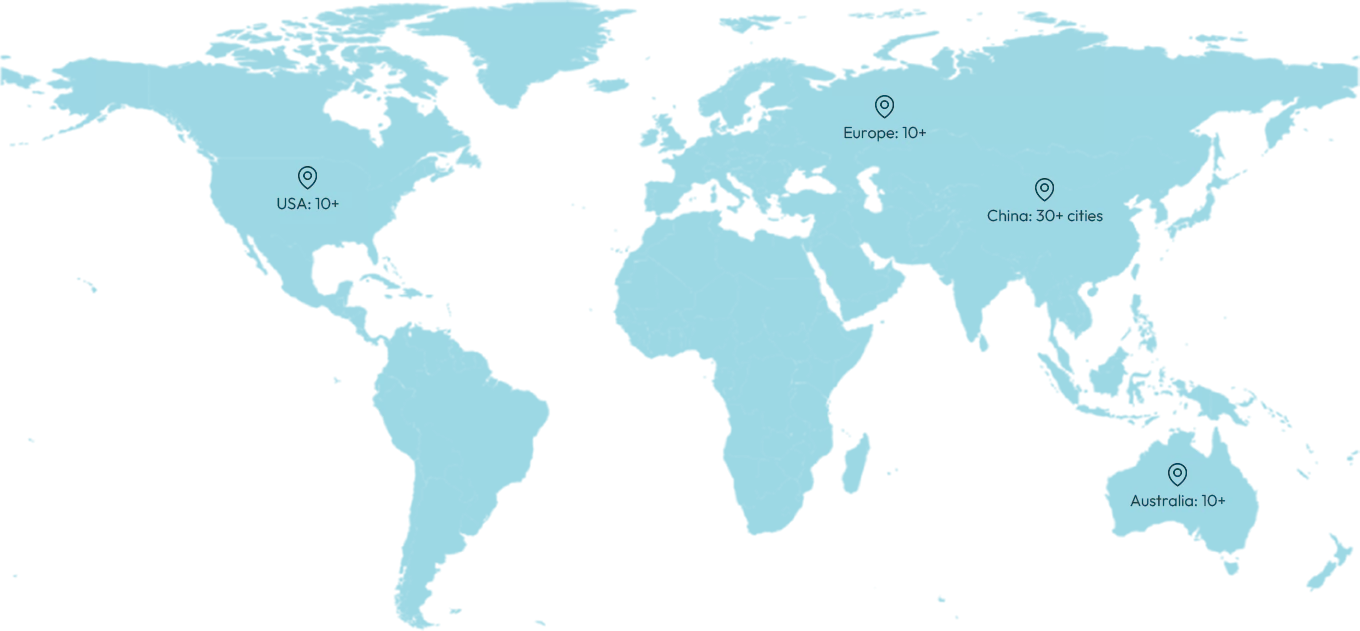
CGT Collaboration Sites
Comprehensive Cell & Gene Therapy (CGT) Clinical Development Services

GCP ClinPlus offers end-to-end CGT clinical development services aligned with global regulatory standards. We have supported 80+ CGT programs, including 40+ in oncology, rare diseases, autoimmune conditions, and regenerative medicine.
Our expertise spans cutting-edge modalities such as CAR-T (targeting BCMA, CD19, etc.), stem cell therapies, and gene therapies using viral vectors (e.g., lentivirus, AAV). Key indications include hematologic and solid tumors, aGVHD, β-thalassemia, retinal diseases, and immune disorders.
We provide full-process risk control with graded SOPs managing CRS/ICANS toxicity—covering tumor burden assessment before infusion, 24/7 multidisciplinary monitoring during treatment, and dynamic cytokine surveillance post-infusion.
Our in-house digital platform enables daily/weekly review and generates FDA/EMA-compliant statistical outputs, reducing data analysis time by up to 30%.
With a global perspective, our dual China–US regulatory teams ensure synchronized submissions and GLP-compliant sample management across international trial sites. Our strong KOL network and 160+ partner sites further ensure rapid startup and quality delivery.
From early-phase studies to pivotal trials, we deliver customized, efficient solutions to accelerate CGT innovation and market entry.
GCP ClinPlus operates across 30 cities in China, as well as in Australia, the U.S. and the EU, offering cross-regional regulatory coordination and clinical execution through our local teams, overseas branches, and CRO partners.

GCP ClinPlus delivers exceptional expertise with genuine commitment to our projects. Their team demonstrates remarkable responsibility at every stage, consistently going above and beyond. In an industry where dedication matters, they've proven themselves an invaluable partner for our success.

After enrollment challenges with our previous CRO threatened our study timeline, GCP ClinPlus stepped in and transformed our project. Their team rapidly accelerated patient recruitment beyond our expectations and put our critical study back on track. Their clinical expertise and swift implementation proved decisive in securing our study's success.

GCP ClinPlus stands out for their exceptional responsiveness and customer focus. Their team consistently prioritizes our needs with prompt, thoughtful solutions to every challenge. Their combination of technical excellence and client-centered approach makes them a truly strategic partner in our development programs.


