In our previous issue, we decoded the evolution of core terminology in the 2025 draft GCP, revealing a transformation in underlying principles. This time, we turn our focus to the "gatekeeper" of clinical trials—the Ethics Review Committee (EC). The new version of the GCP significantly reshapes and optimizes the responsibilities of the EC. These changes are not only about efficiency but also profoundly reflect a more precise, science- and risk-management-based mindset.
What are the specific changes? The chart below will clearly illustrate the key points for you.
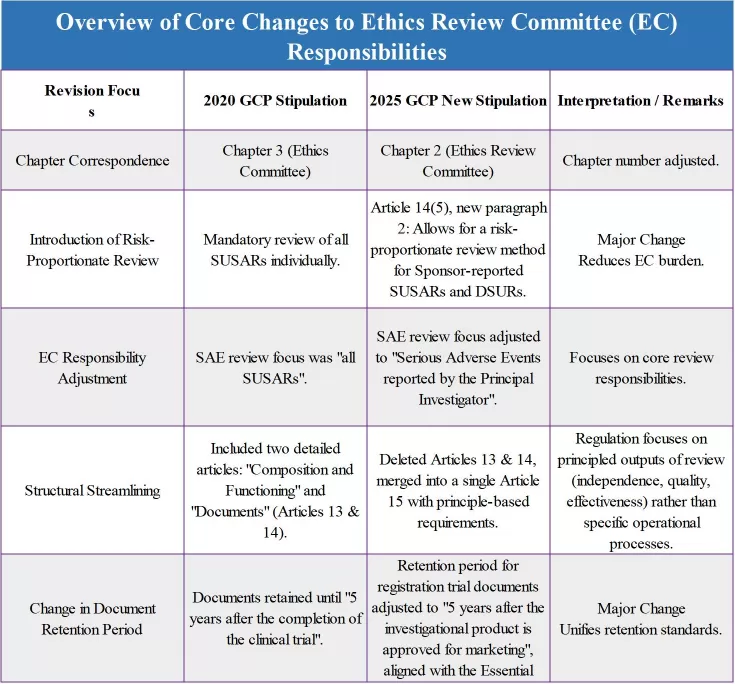
These revisions to the Ethics Review Committee precisely address long-standing pain points in the industry. By introducing risk-proportionate review, it unlocks review capacity; through responsibility adjustments, it clarifies the EC's core review duties; and by unifying standards and mitigating risks, it resolves the issue of potential premature expiration of EC documents due to long drug development cycles. Collectively, these changes aim at one goal: building a more efficient, scientific, and clearly accountable ethical review system. This empowers the "gatekeeper" to focus its efforts on fortifying the most critical line of defense for the rights, safety, and well-being of trial participants.
With the responsibilities of the Ethics Review Committee now clarified, what key changes have occurred to the responsibility boundaries of the Principal Investigator (PI), who bears the ultimate responsibility at the research site frontline? How does the new GCP use clear clauses to outline a definitive "list of ultimate responsibilities" for the PI? Stay tuned for tomorrow's article: The PI Chapter, where we will analyze this point by point!