We are excited to launch our "GCP Insights – 7-Day Series", a deep dive into China's newly released "Drug Clinical Trial Quality Management (GCP) 2025 Draft for Comments", published by the National Medical Products Administration (NMPA) on October 25, 2025.
As a cornerstone of drug development regulation, each revision of the GCP signals a significant shift in China's clinical trial quality management system.
In this opening article, we focus on the structural and key clause changes between the 2025 draft and the 2020 version. Use the comparison below to quickly grasp the revision's core direction:
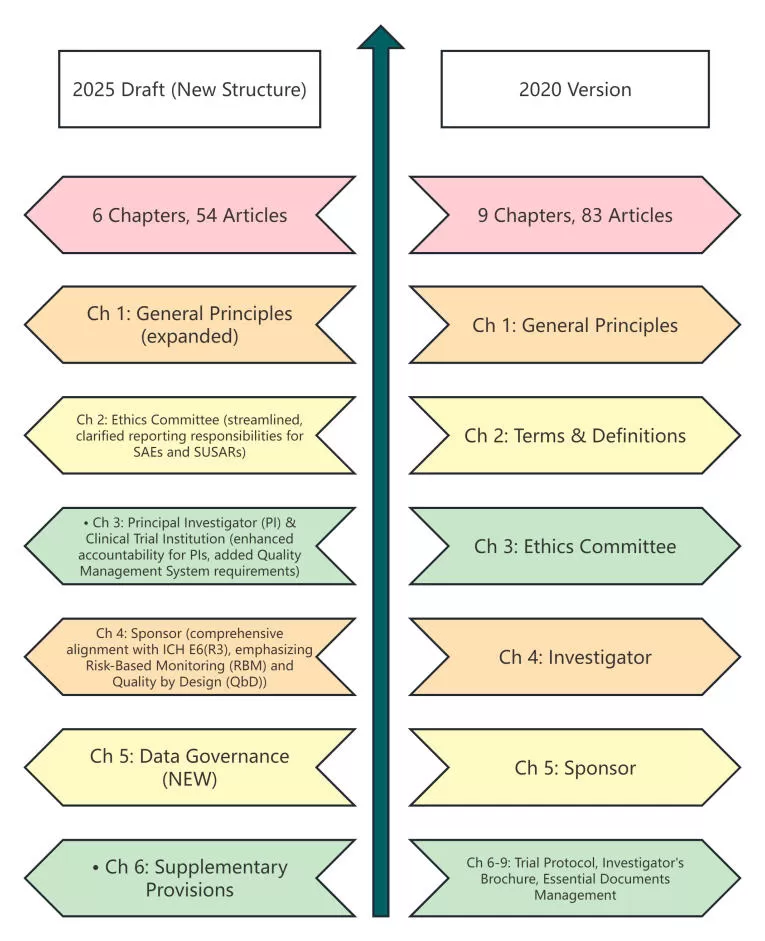
Key Takeaways:
✓Streamlined Framework: The consolidation from 9 to 6 chapters signifies a move towards a more principle-based approach, with clearer role definitions.
✓New Chapter on Data Governance: A dedicated chapter underscores the critical importance of data integrity and oversight in the digital era.
✓Strengthened Responsibilities: Roles of the Sponsor and Principal Investigator (PI) are reinforced, fully aligning with ICH E6(R3) concepts. Requirements for service providers are now equivalent to those for the Sponsor.
✓Deleted Sections: Standalone chapters on the Trial Protocol, Investigator's Brochure (IB), and Essential Documents Management have been removed, as these are now comprehensively covered under the adopted ICH E6(R3) framework.
These changes reflect China's decisive move toward a modernized, risk-based, quality-focused, and accountability-driven clinical trial governance system.
What's Next in the Series:
Stay tuned for Day 2: "Is the 'Ethics Committee' Out? 6 New Terms You Must Know"
