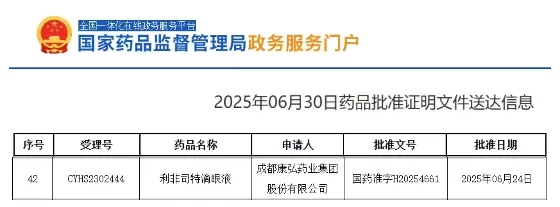
When dry eye patients suffer from recurring dryness and stinging, the launch of a new drug could be the key to transforming their quality of life. Recently, a major milestone emerged in China's dry eye treatment landscape—Conber Pharma's Category 3 new drug, lifitegrast ophthalmic solution (Langyueming®), has been officially approved for exclusive market launch, bringing new therapeutic hope to millions of dry eye patients. Behind this milestone achievement, Pristine Pharma, as Conber Pharma's long-term partner, participated comprehensively throughout the process, empowering the entire R&D lifecycle with professional services and leveraging data science to drive the innovation from lab to clinic.
From project initiation to final approval, Pristine's team worked hand-in-hand with Conber Pharma, deeply integrated into the full-indication R&D process of lifitegrast ophthalmic solution. To meet the project's complexity and high demands, we rapidly assembled a premier team—spanning 12 critical project roles with 49 experts in data management, biostatistics, and clinical programming collaborating across disciplines. This ensured precision and control at every stage, from data collection and cleaning to statistical analysis and report generation.
On the R&D journey, data is the lifeline. Our team invested 3,548.4 hours combing through massive datasets, rigorously validating every value and report with meticulous logic. Facing critical milestones, team members voluntarily contributed over 120 hours of overtime, breaking through progress barriers with agile responsiveness. Ultimately, we facilitated 10 major submissions, laying a robust data foundation for the smooth approval of the New Drug Application (NDA).
The launch of a new drug is never accidental. The successful approval of lifitegrast ophthalmic solution fills a clinical gap in dry eye treatment—a testament to Conber Pharma's R&D capabilities and Pristine's professional expertise in innovative drug development services. We are not just data custodians but R&D enablers: optimizing study design through scientific statistics, shortening development cycles via efficient data management, and supporting decisions with precise analytical outcomes—all to accelerate patient access to innovative therapies.
As a leader in China's pharmaceutical CRO sector, Pristine Pharma has built formidable expertise through deep industry experience and a philosophy of "scientific rigor, efficiency, and innovation." Our successful delivery of over 2,200 clinical studies stands as proof of our ability to empower pharma R&D and earn widespread trust. Pristine remains unwavering in its commitment: to fuel medical innovation with cutting-edge expertise, bringing medical breakthroughs to more patients worldwide.
PREV