With the further implementation of the new GCP, the central figure in clinical trials—the Principal Investigator (PI)—is facing more defined and stringent responsibility guidelines. The chart below will clearly illustrate the key revisions!
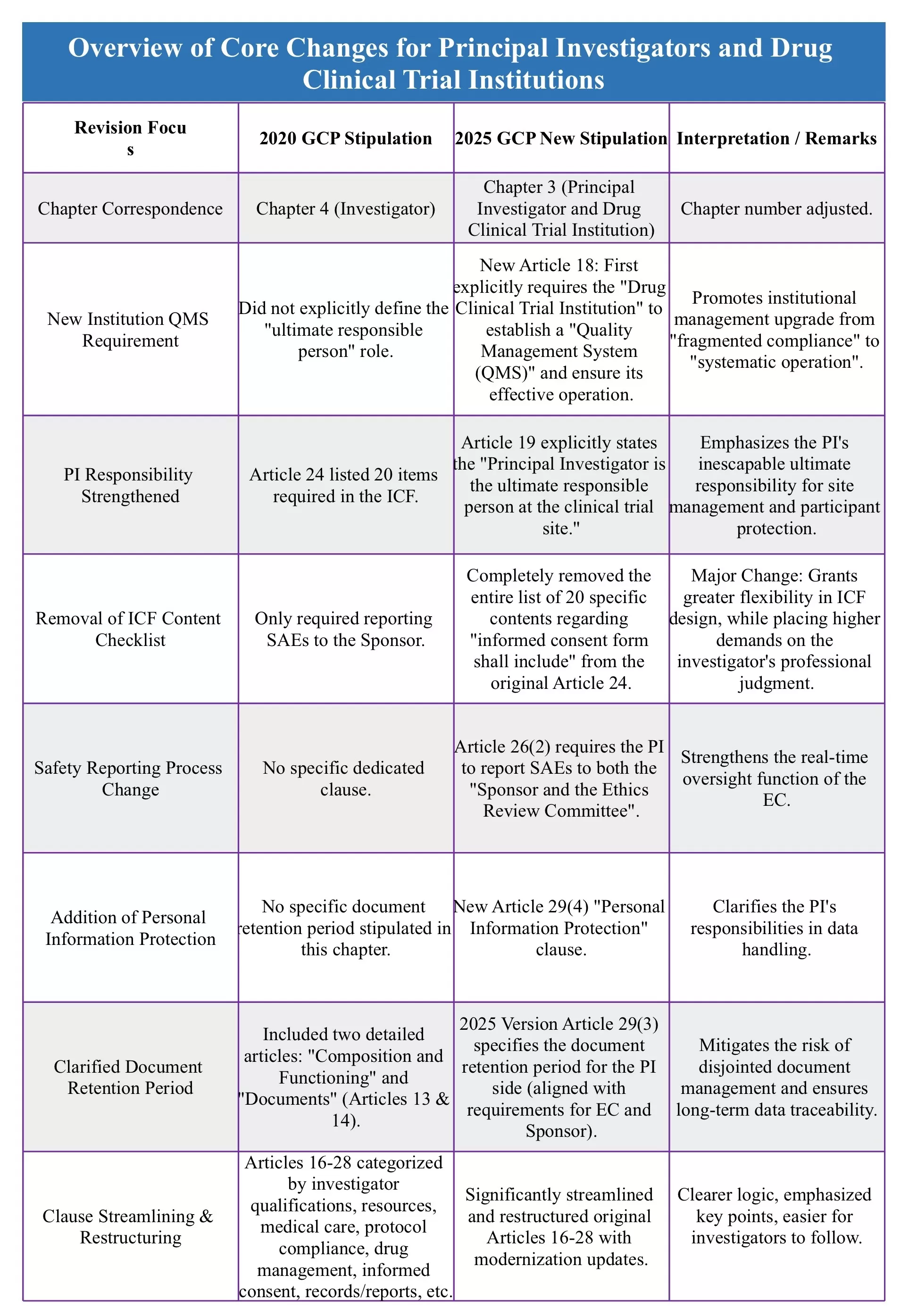
Through a systematic reshaping of PI responsibilities, the new GCP fundamentally addresses the potential issues of "passive execution" or "unclear authority and accountability." The evolution from the "primary responsible person" to the "ultimate responsible person" is not merely a change in wording but a significant reinforcement of accountability. This series of changes aims to establish an on-site execution environment characterized by commensurate authority and responsibility, as well as unified standards, enabling PIs to genuinely fulfill their core mission of safeguarding participant safety and data quality.
Now that the responsibilities of the Ethics Committee and the Principal Investigator have been clarified, how has the responsibility framework for the Sponsor—the core entity that initiates and manages clinical trials—been restructured? The upcoming analysis covers the most significantly revised chapter in the new GCP: the comprehensive introduction of Risk-Based Management (RBM) and Quality by Design (QbD). Why were the detailed clauses on monitoring and auditing removed, replaced by an emphasis on Root Cause Analysis and Corrective and Preventive Actions (RCA/CAPA)? The answers will be revealed in tomorrow's article: The Sponsor Chapter.