After six days of layered analysis, we finally arrive at the concluding piece of the "GCP Insights · Seven-Day Series". The revisions in the GCP 2025 (Draft for Comment) represent not just an update to the text, but a deep restructuring of the underlying regulatory logic. Today, we focus on the most strategically significant shift in this revision – the streamlining and integration of the chapter framework.
The specific changes to the chapters are outlined as follows:
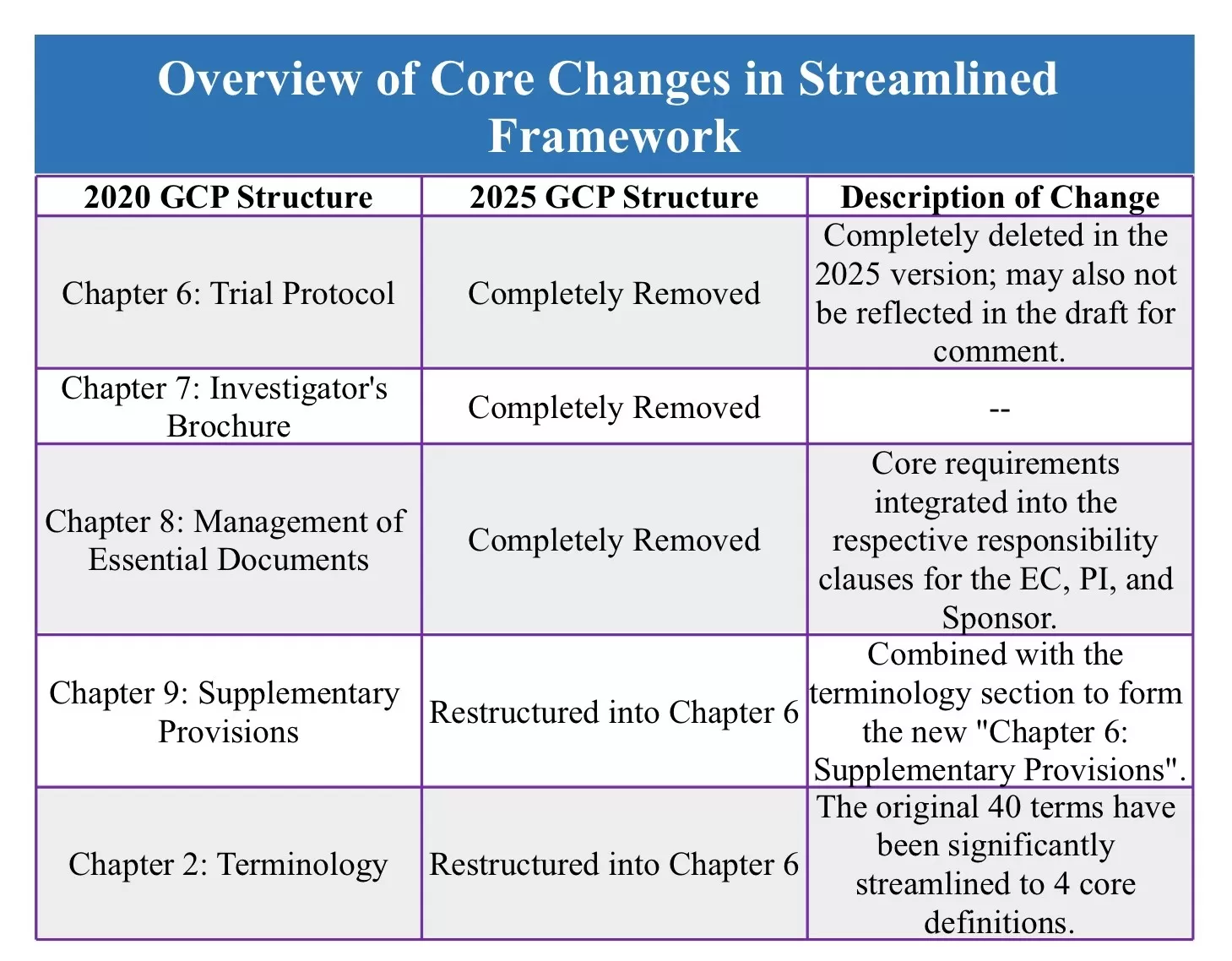
This streamlining of the GCP 2025 (Draft for Comment) framework is by no means a simple deletion of clauses; it is a fundamental upgrade in regulatory thinking. On one hand, the removal of independent chapters for the trial protocol, investigator's brochure, etc., and the dispersal of their core requirements into the respective responsibility clauses for different parties, resolves past issues of overlapping clauses and ambiguous responsibility definitions. This makes the obligations of entities like Sponsors and Investigators clearer and more actionable. On the other hand, the significant simplification of the terminology chapter and the restructuring of supplementary provisions strip away regulatory complexity, making the text more aligned with the practical needs of clinical trials in a digital and international context.
This adjustment not only fully aligns with the core requirements of ICH E6(R3) but also lays an institutional foundation for the international recognition of clinical trial data from China, facilitating future trust from international regulatory agencies like the EMA and FDA. Under this clearer regulatory framework, the industry is expected to achieve a dual advancement in quality control and innovative development, propelling China's drug R&D steadily towards high quality and internationalization.
With this, the "GCP Insights · Seven-Day Series" concludes successfully. Thank you for your sustained attention over these seven days. We hope this series has provided you with strong support in understanding the new regulations of GCP 2025 (Draft for Comment).