Developing CGTs is incredibly complex and expensive. Global sponsors face regulatory hurdles, manufacturing complexities, and patient recruitment challenges that can derail even the most promising therapies.
Partnering with an experienced China-based CRO like GCP ClinPlus provides global sponsors with access to vast patient pools, specialized regulatory expertise, and integrated services, accelerating Cell and Gene Therapy development and approval in key markets like China, the US, and Europe.
The potential is clear, but how exactly does this partnership translate into tangible benefits for your specific CGT program? Let's break down the critical advantages.
Patient recruitment is the single biggest bottleneck in clinical trials. For rare diseases targeted by CGTs, finding enough qualified patients can take years, burning through your budget.
A China CRO leverages the country's vast and genetically diverse patient population. With established site networks and deep therapeutic knowledge, they can dramatically shorten recruitment timelines, getting your trial on track faster.
Beyond Just Numbers: A Strategic Recruitment Advantage
Simply having access to a large population isn't enough. The real acceleration comes from a strategic, data-driven approach tailored to CGTs.
Therapeutic Area Focus: GCP ClinPlus has a dedicated CGT business unit with a proven track record. This means pre-vetted sites and investigators who understand the unique protocols and patient management requirements of advanced therapies.
Integrated Site Management: Our model goes beyond identifying sites. We provide comprehensive site support, including training on complex procedures, managing logistics for cell shipment, and ensuring adherence to strict chain-of-custody protocols, which is critical for CGT success.
Data-Driven Insights: We utilize proprietary tools to analyze patient databases and identify potential candidates more efficiently, moving beyond traditional recruitment methods.
| Traditional Recruitment Challenge | GCP ClinPlus' Strategic Solution |
| Lengthy site identification and activation | Pre-established network of CGT-experienced sites |
| Difficulty in recruiting for rare indications | Access to a vast, treatment-naive population across a wide genetic spectrum |
| Managing complex CGT logistics at sites | Integrated site management and training for protocol-specific procedures |
This multi-faceted approach turns patient recruitment from a bottleneck into a strategic accelerator.
CGT trials aren't just "drug" trials. They combine clinical operations with complex logistics, manufacturing, and stringent regulatory oversight. A standard CRO model often falls short.
Integrated support means a single provider manages the entire lifecycle: from regulatory strategy and site selection to clinical operations, biometrics, and critical logistics like cell transport and storage, ensuring seamless execution.
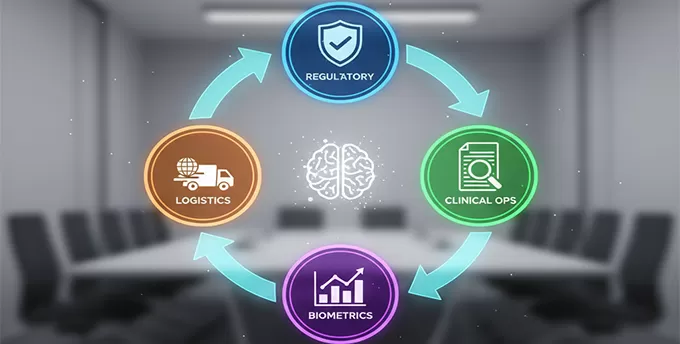
Deconstructing "Full-Service" for CGT Success
A siloed approach creates risk and inefficiency. For CGTs, integration is non-negotiable. Our support is built on four interconnected pillars:
Regulatory Strategy and Submissions: We navigate the complex web of NMPA (China), FDA, and EMA requirements simultaneously. Our team has a history of successful IND and BLA submissions, understanding the nuanced differences between agencies to create a cohesive global strategy.
Clinical Operations and Site Management: Our therapeutic experts design and manage protocols with a deep understanding of CGT endpoints and patient safety monitoring. We ensure sites are not just activated, but fully empowered.
Biometrics and Data Management: With over 110 proprietary tools, we provide advanced data management, statistical analysis, and programming. This ensures data integrity and quality, ready for regulatory submission.
Specialized CGT Logistics Coordination: We have the processes and partners to manage the vital handoffs between clinical sites, manufacturing facilities, and testing labs, addressing the unique cold-chain and timing challenges of living therapies.
This holistic model eliminates finger-pointing between vendors and provides you with a single point of accountability, reducing complexity and mitigating risk throughout the trial's duration.
Partnering with a specialized China CRO like GCP ClinPlus accelerates CGT development through faster recruitment, integrated expertise, and a clear path to global regulatory success.