In the previous issue, we provided a macro-level analysis of the structural changes in the 2025 draft GCP (solicitation for comments), highlighting the evolution of the regulatory framework toward a 'risk-based, quality-first, and responsibility-clarified' approach. However, the true transformation often lies in the details. In this issue, we will focus on the updates to core terminology in the text, decoding the profound evolution of China's clinical trial governance philosophy.
The revision of terminology is by no means a mere word game but rather the crystallization of evolving regulatory thinking, ethical concepts, and industry practices. The table below clearly illustrates the iteration of key terms:
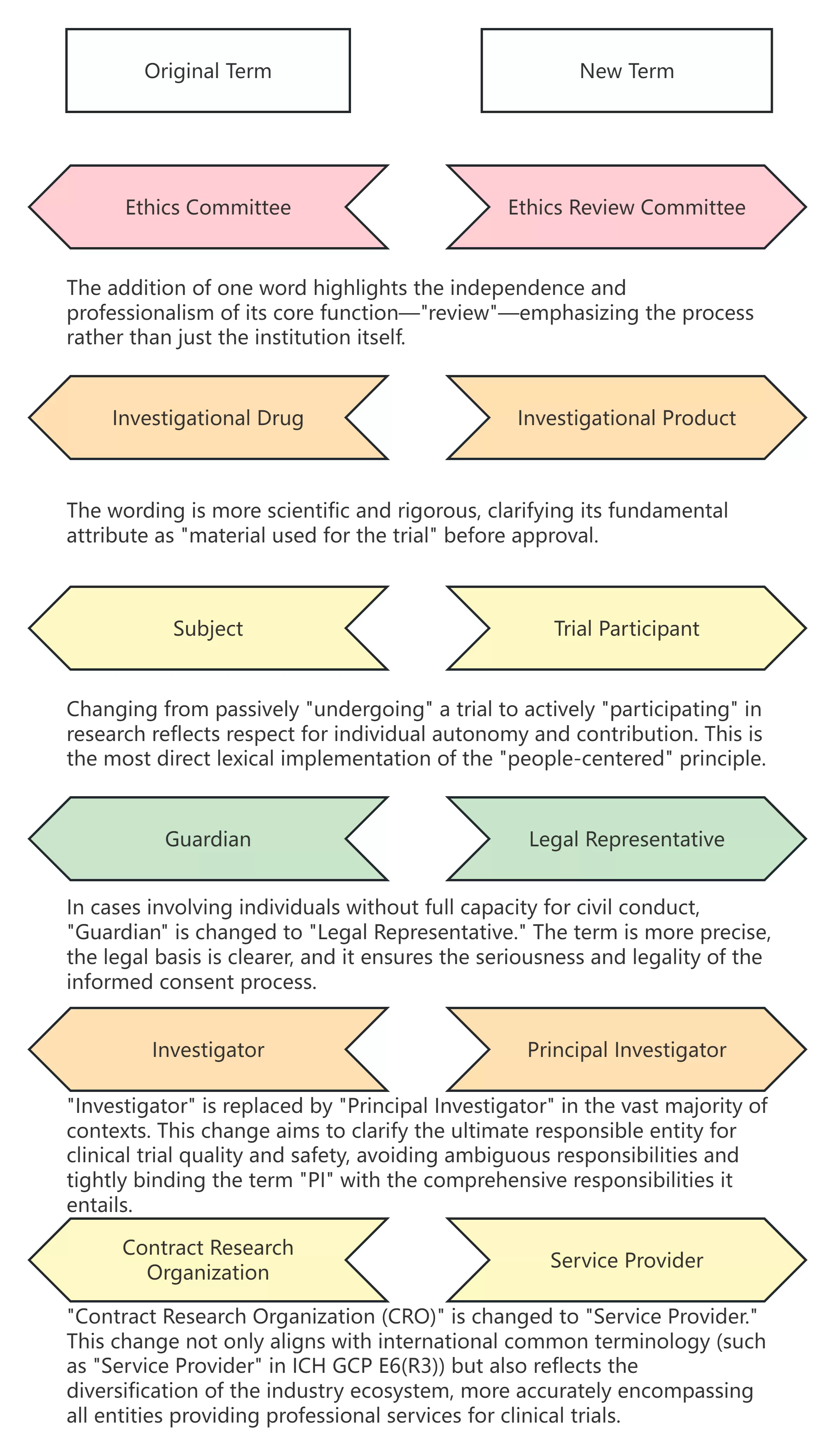
The evolution of terminology is the clearest indicator of China's clinical trials entering a new phase of 'people-centered, clear division of responsibilities, and international alignment.' Accurately understanding and actively adapting to these changes is precisely the prologue we must draw to open a new chapter in GCP regulation.
In the next issue, we will delve into another significant revision: the Ethics Review Committee. Stay tuned!

