When the entire chapter on "Monitoring" and "Auditing" has almost disappeared from the new GCP, how should Sponsors respond? This is not a regulatory retreat, but a profound shift from "how to do" to "why to do." In this issue, we will deeply interpret the reconstruction path of the Sponsor's responsibility system, examining how quality management is being upgraded from mechanical compliance to intelligent construction. The chart below clearly presents the key revisions!
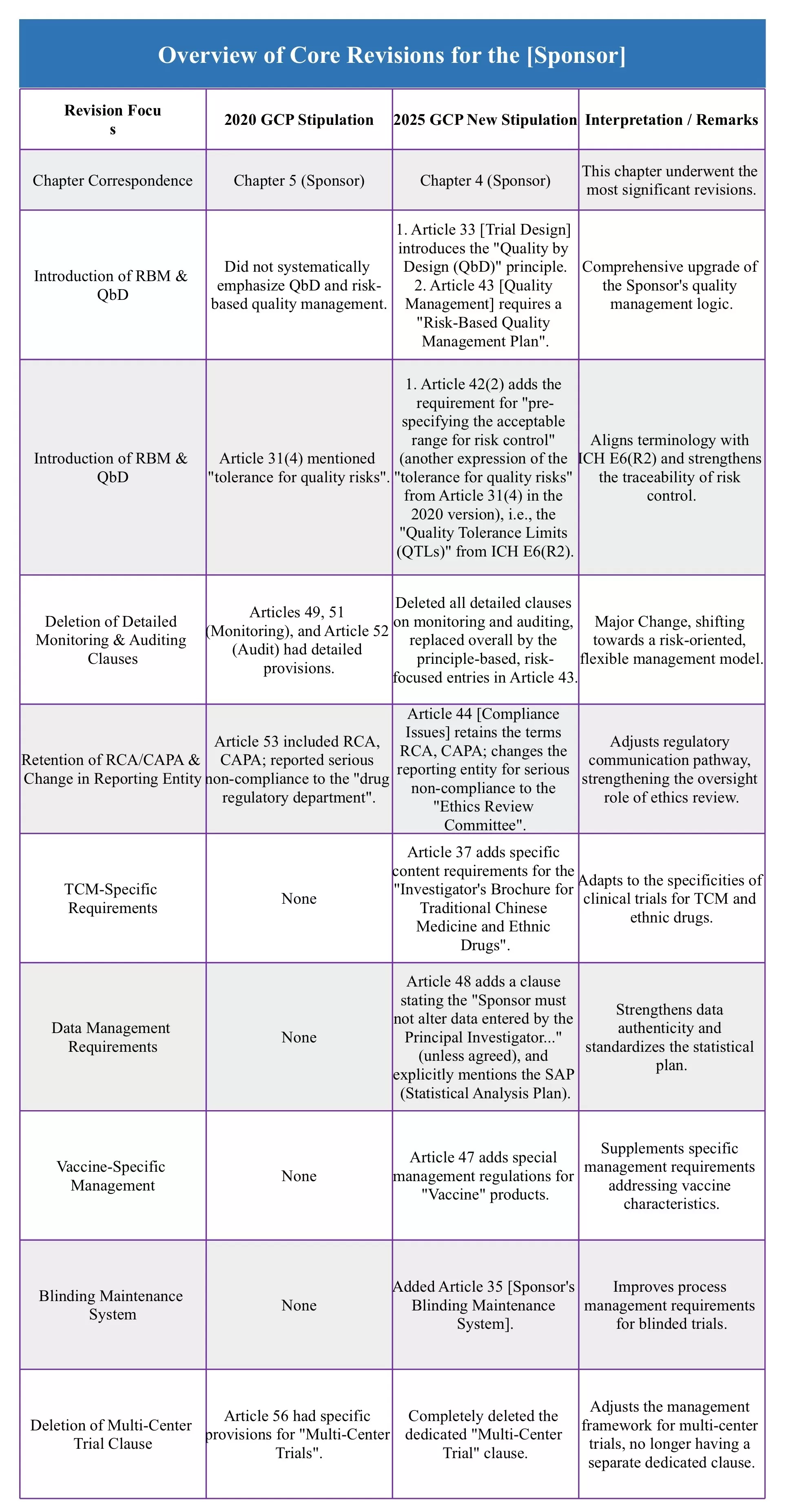
The revisions to the Sponsor chapter in the new GCP represent a deep innovation in quality management philosophy. The "disappearance" of detailed monitoring and auditing clauses does not signal lower requirements, but rather an evolution in concept. It forces Sponsors to move away from relying on rigid compliance rules and instead focus on building a dynamic, efficient, and proactive quality management system centered on critical data and quality. The direction indicated by the new GCP for Sponsors is clear: your quality originates from your design; your risks require your management.
With the Sponsor's responsibility system now reconfigured, what fundamental changes have occurred to the rules governing data—the cornerstone of clinical trial evidence? Tomorrow, we will delve into a brand-new chapter: "Data Governance", exploring how the GCP responds to the demands of the digital age by establishing rules for electronic data management and the maintenance of blinding.